
In the production of fine chemicals and the electronics industry, ultrapure water must be strictly produced according to established standards. This is essential to ensure the quality standards of fine chemical products and the integration of circuits. During the production phase, there are high purity requirements for raw materials, and strict production standards must also be followed for other chemical components and gas purity. Moreover, ultrapure water plays a crucial role for production enterprises.

Sepurlite supplies various resin types for ultrapure water treatment application for water softening, water desalination and water polishing. They can be used combined with a series system to guarantee the final water quality and ensure the smooth and efficient industrial production.
Ion exchange resins are widely used in the ultrapure water treatment for water softening, desalination and polishing. Different water quality requirements requires different treatment system, we can design the suitable treatment system for your ultrapure water production.
Ion exchange technology can indeed be used for water softening during the pretreatment stage of ultrapure water processing. The main goal of water softening is to remove hardness ions from the water, particularly calcium ions (Ca2+) and magnesium ions (Mg2+), in order to prevent scale buildup and protect subsequent treatment equipment (such as reverse osmosis membranes) from scaling damage.
Working Principle
In the water softening process, cation exchange resins are commonly used, typically in sodium ion (Na+) form. When hard water passes through the resin bed, the calcium ions (Ca2+) and magnesium ions (Mg2+) in the water exchange with the sodium ions on the resin, thereby removing the hardness ions from the water and replacing them with sodium ions. This process effectively reduces the hardness of the water and prevents scale formation. The reaction is as follows:
R-Na + Ca2+ → R-Ca + 2Na+
R-Na + Mg2+ → R-Mg + 2Na+
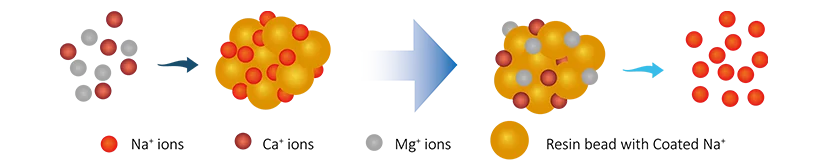
During this process, the ion exchange resin captures calcium and magnesium ions from the water, preventing them from forming precipitates in subsequent treatment equipment.
Ultrapure water mixed-bed polishing is a critical step in the production of ultrapure water, typically serving as the final stage of the water treatment process to further remove residual trace ions, thereby achieving extremely high purity requirements. Mixed-bed polishing is particularly important in fields such as semiconductor manufacturing, pharmaceuticals, optics, and the nuclear industry, where water quality demands are extremely high.
Working Principles
The mixed-bed polishing system contains both cation exchange resins and anion exchange resins, which are mixed in the same container (i.e., the mixed bed). Its operating principle utilizes the ion exchange functionality of the resins to exchange the remaining cations and anions in the water with hydrogen ions (H+) and hydroxide ions (OH-) on the resins, ultimately generating water molecules (H2O). This process significantly reduces the water's conductivity, allowing the water purity to meet ultrapure water standards. The reaction is as follows:
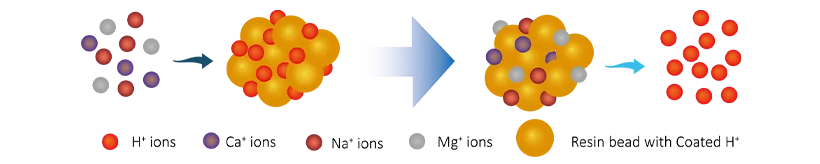
Cation resins reaction
R-H + Na+(Ca2+/Mg2+) → R-Na(Ca2+/Mg2+) + H+
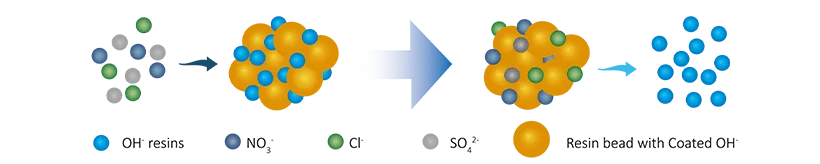
Anion resins reaction
R-OH + Cl-(SO42-/NO3-/HSiO3-) → R-Cl(SO42-/NO3-/HSiO3-) + OH-
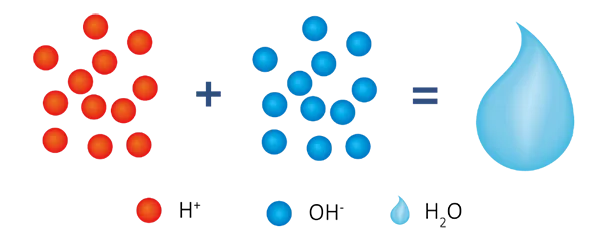
Final reaction
R-H + R-OH +Na+ (Ca2+/Mg2+) + Cl-(SO42-/NO3-/HSiO3-) → R-Na + R-Cl + H2O
Ultrapure water plays a significant role in several high-tech industries, including:
What is Ultra Pure Water?
Ultrapure water refers to water with extremely high purity, typically having a conductivity of less than 0.055 μS/cm and a resistivity of 18.2 MΩ·cm or higher. Common specifications call for a TOC (total organic carbon) of lower than 5 µg/L (ppb). This water contains virtually no ions, organic compounds, microbes, or particulate matter, making its quality considered pollution-free. The production and use of ultrapure water are crucial in many high-tech industries.
Why We Need Ultra Pure Water?
Ultrapure water is widely used in the high-tech industries where there have ultra strict requirement of water quality. In the production of integrated circuits, semiconductor chips and liquid crystal or plasma displays, water of extreme purity is required in certain processing steps. Integrated circuits contain very fine conductors, spaced by a fraction of a µm. Any minute impurity sitting across two conductors will create a short-circuit and ruin the whole device. The closer the distance between the conductors, the more drastic is the required purity of water used in the manufacturing process.
Some other demand for ultrapure water arises from several factors:
How to Produce Ultra Pure Water?
The production of ultrapure water typically involves several steps, including:

No matter guides, inquiry or assistance, our experts are ready to serve you.