Ion Exchange Resin Definition & Category
Introduction
Ion exchange resins are solid, high molecular weight materials, typically in the form of small beads, that possess the ability to exchange ions in a solution. These resins are capable of swapping specific ions in the solution with ions that are present on the resin itself. Ion exchange resins are widely used for water treatment, chemical, and other industrial processes, where selective ion exchange is necessary to purify, separate, or concentrate certain substances.
Structure
- Polymer Matrix: The backbone of the resin, typically made from polystyrene, polypropylene, or other synthetic polymers. This matrix provides physical stability and supports the resin's overall structure. The polymer matrix is usually spherical or granular in form and is designed to have a large surface area for efficient ion exchange.
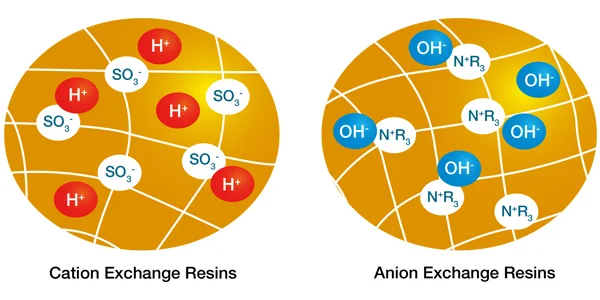
- Functional Groups: These are the active components of the resin responsible for ion exchange. The functional groups are typically either acidic or basic in nature, and they are chemically bound to the polymer matrix.
- Cation Exchange Resins: Have acidic functional groups (e.g., sulfonic acid groups SO3-) that can exchange cations (positively charged ions) like sodium (Na+), calcium (Ca2+), or magnesium (Mg2+).
- Anion Exchange Resins: Have basic functional groups (e.g., quaternary ammonium groups N+R3) that can exchange anions (negatively charged ions) like chloride (Cl-), sulfate (SO42-), or fluoride (F-).
Classification
Ion exchange resins are classified based on several criteria, such as their ion exchange properties and the type of ions they exchange, and the strength of their functional groups.
- Based on Ion Exchange Type
- Cation Exchange Resins: These resins exchange cations (positively charged ions), such as sodium (Na+), calcium (Ca2+), magnesium (Mg2+), or heavy metals like lead (Pb2+). They are widely used in water softening, heavy metal removal, and other applications where the removal of cations is needed.
- Anion Exchange Resins: These resins exchange anions (negatively charged ions), such as chloride (Cl-), sulfate (SO42-), or nitrate (NO3-). They are used to remove harmful anions from water or solutions, such as removing sulfate ions in desalination processes or fluoride ions in water purification.
- Based on the Strength of Functional Groups
- Strong Acid Cation Exchange Resins: These resins contain strong acidic functional groups, such as sulfonic acid groups (-SO3H), which dissociate completely in water and allow for high ion exchange capacity across a wide pH range (from strongly acidic to neutral). They are commonly used in water softening and removing heavy metals from solutions.
- Weak Acid Cation Exchange Resins: These resins contain weak acidic functional groups, such as carboxylic acid groups (-COOH), which only partially dissociate in water. These resins are effective in milder, slightly acidic environments and are typically used in more specific applications, like selective removal of calcium or magnesium ions in less demanding conditions.
- Strong Base Anion Exchange Resins: These resins contain strongly basic functional groups, such as quaternary ammonium groups (-NR3+), which dissociate completely in water and are capable of exchanging anions over a broad pH range. They are widely used for the removal of anions like chloride, sulfate, and nitrate.
- Weak Base Anion Exchange Resins: These resins contain weaker basic functional groups, such as amine groups (-NH2), which only partially dissociate in water and are effective in slightly alkaline or neutral conditions. They are often used for the selective removal of weakly acidic anions.
Ion Exchange Process
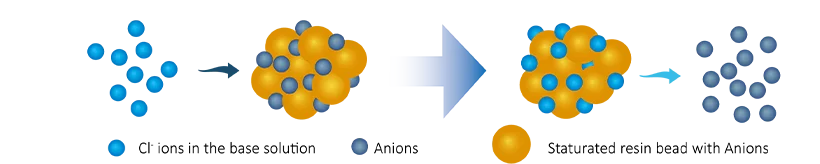
When the solution containing undesirable ions passes through the resin, the ions in the solution (e.g., calcium or magnesium) are attracted to the resin's functional groups. The resin releases an equivalent amount of ions (e.g., sodium or hydrogen ions) into the solution in exchange.
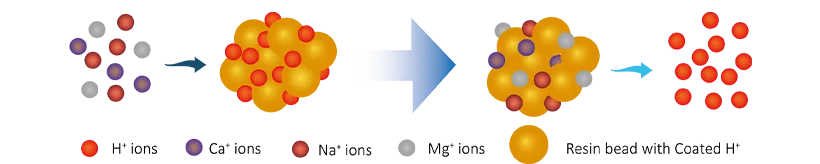
- Cation exchange. The dissolved cation ions (Ca2+, Mg2+, Na+) will be exchanged with H+, the ion exchange process is as follows:
R-H + Na+ → R-Na + H+
R-H + Ca2+ → R-Ca + 2H+
R-H + Mg2+ → R-Mg + 2H+
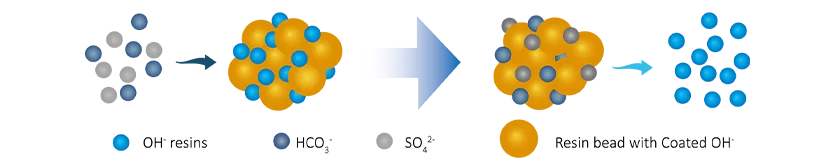
- Anion exchange. The dissolved cation ions (Cl-, SO₄2-, CO₃2-) will be exchanged with OH-, the ion exchange process is as follows:
R-OH + Cl- → R-Cl + OH-
2(R-OH) + CO32- → (R2CO3) + 2OH-
2(R-OH) + SO42- → (R2SO4) + 2OH-
Regeneration Process
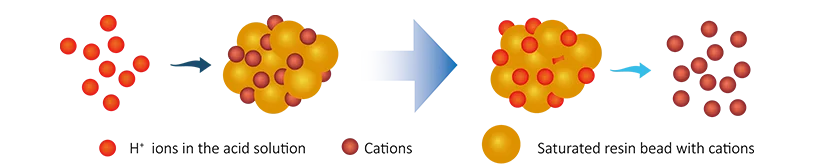
After a period of use, the ions on the resin are gradually exhausted and regeneration process is required.
- Cation exchange resin regeneration: Hydrogen ions are restored to the resin by regeneration using an acid solution such as hydrochloric acid HCl or sulfuric acid H2SO4.
- Anion exchange resin regeneration: Regeneration using a base solution (e.g. sodium hydroxide NaOH) restores the hydroxide ions of the resin.
Characteristics
- High Ion Exchange Capacity: Ion exchange resins have a high capacity for exchanging ions, which is determined by the number of exchange sites and the type of functional groups present. This makes them suitable for large-scale water treatment and chemical separation processes.
- Selectivity: Ion exchange resins exhibit high selectivity, meaning they can preferentially exchange specific ions depending on their size, charge, and chemical nature. This property is crucial for applications such as water softening, removing trace elements, or purifying chemicals.
- Regenerability: Ion exchange resins can often be regenerated by using specific chemical solutions to replace the ions that were exchanged during the process. This allows the resin to be reused, making it cost-effective and environmentally friendly.
- Chemical and Physical Stability: Ion exchange resins are stable in a wide range of chemical environments, including acidic and basic conditions. They also have good mechanical strength, allowing them to withstand physical stress during use.
Applications
Ion exchange resins are used in various industries for diverse applications, including:
- Water Treatment
- Water Softening: Removing calcium and magnesium ions to prevent scale formation in pipes and equipment.
- Demineralization: Removing all dissolved salts from water, typically in power plants or laboratories.
- Heavy Metal Removal: Removing toxic heavy metals like lead, cadmium, and mercury from wastewater.
- Chemical and Pharmaceutical Industries
- Purification and Separation: Ion exchange resins are used to purify and separate specific chemicals in the pharmaceutical, food, and chemical industries.
- Acid/Base Treatment: Used for neutralizing acidic or basic solutions in various chemical processes.
- Food and Beverage
- Sugar Refining: Removing colorants and impurities from sugar syrups.
- Beer and Wine Processing: Removing undesirable compounds from beverages.
- Nuclear
- Radionuclide Removal: Used to remove radioactive ions from waste streams in nuclear power plants.
- Environmental Protection
- Removing anions and cations from industrial wastewater before discharge or reuse.
- Electronics and Battery Manufacturing
- Pure Water for Electronics: Providing ultra-pure water used in semiconductor manufacturing.
- Battery Reconditioning: Removing impurities from battery solutions.
 Need Any Help?
Need Any Help?
No matter guides, inquiry or assistance, our experts are ready to serve you.