Mixed Bed Resins: The Core of Deionized & Ultra Pure Water
Mixed bed resins are popular in the water treatment industry to produce ultra-pure water and various other applications. Cation resins and anion resins are placed in a container in a specific ratio and they are mixed thoroughly to remove all ions in the water. This article will provide a comprehensive overview of mixed bed resins, covering their definition, structure, classification, working and regeneration process, features, and application.
What are Mixed Bed Resins?
Mixed bed resins refers to resins used in mixed bed system. In this system, cation exchange resins and anion exchange resins are blended in specific proportions and they work together to effectively remove both cations and anions from water, achieving deionization. These resins are extensively used in producing ultrapure water, especially in industries like electronics, pharmaceuticals, and food & beverages, where high water quality is critical.
Structure
The structure of mixed bed resins primarily consists of two components:
- Cation Exchange Resins: Typically composed of polystyrene-based polymers, cation resins are treated with acids or bases to form active ion-exchange sites. These sites interact with cations in water (such as Ca2+, Mg2+, Na+), enabling the removal of positively charged ions.
- Anion Exchange Resins: Similarly made from polystyrene-based polymers, anion resins undergo ionization treatment to form sites capable of exchanging negatively charged ions. These sites effectively remove anions from water (such as HCO3-, SO42-, Cl-, and NO3–).
The uniform blending of these two resin types make exchange process repeat in thousand times to produce high quality pure water.
Classification
Mixed bed resins can be classified into different categories depending on application requirements and resin types:
- Strong Acid Cation (SAC) Exchange and Strong Base Anion (SBA) Exchange Resins: The most popular and widely used combination, they can effectively removing a broad range of ions.
- Weak Acid Cation (WAC) Exchange and Weak Base Anion (WBA) Exchange Resins: They are tailored for specific water treatment needs, such as selectively removing certain organic materials. They are commonly used in applications requiring precise water quality control.
Classical Models
Working Principle
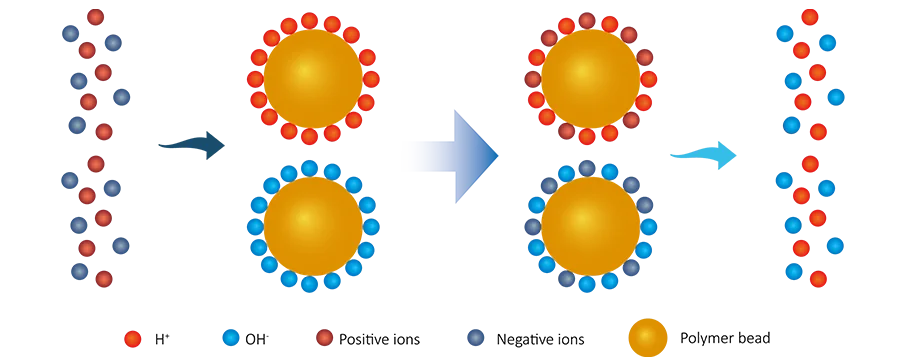
The working principle of mixed bed resins is based on ion exchange reactions.
As raw water enters the vessel, and flows through the mixed bed. The raw water consists of positive (Ca2+, Mg2+, Na+) and negative (HCO3-, SO42-, Cl–, NO3–) ions and the mixed bed vessels has cation (commonly with H+ ions) and anion (commonly with OH- ions) resins.
All positive ions in the raw water are replaced by the cation resins with the H+ form and release H+ ions.
R–H + Ca2+ → R–Ca + H+
At the same time,
All negative ions in the raw water are replaced by the anion resins with the OH- form and release H+ ions.
R–OH + NO3- → R–NO3 + OH-
Same manner, both anion, and cation resins release OH– and H+ ions, and deionized water will be generated.
Regeneration Process
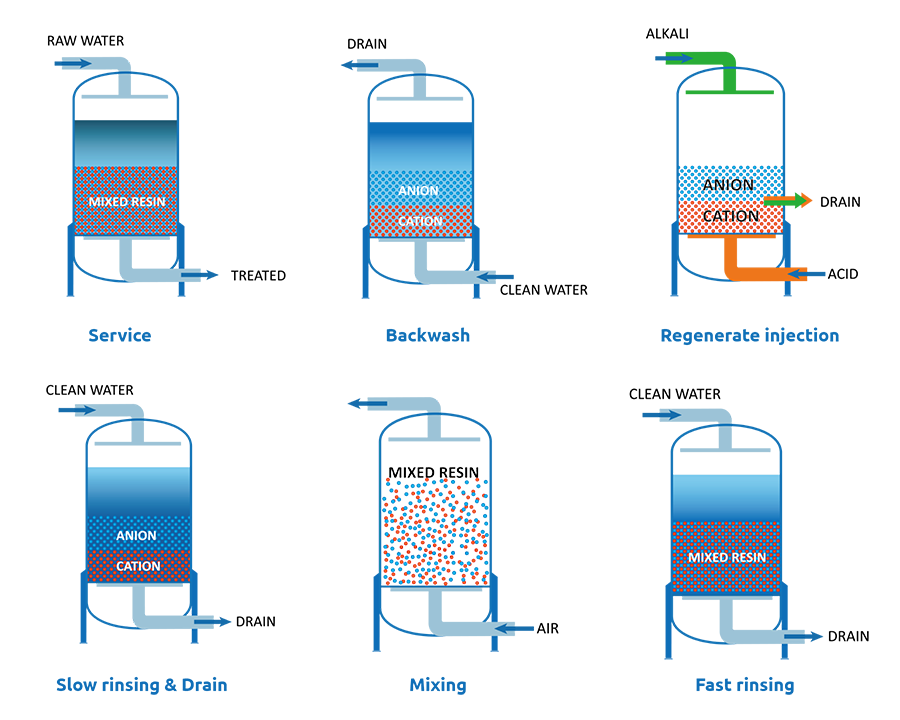
Over time, mixed bed resins lose their ion exchange capacity and require periodic regeneration. The regeneration process generally includes the following steps:
- Backwashing: Backwash the failed resins to separate them into separate cation and anion layer due to different density of cation and anion resins. Cation resins have a higher density and they will goes down to bottom of the vessel during backwashing while the anion resins will goes upstream to the top of the vessel. You may adjust the backwash flow properly to guarantee the completely separate and in case of escaping at the same time. Additional, the flow rates should ensure the mixed bed have enough expansion space. After backwashing, the cation and anion resins need a few minutes to settle into separate layers until there is a sharp separation line and no more cross mixing of anion and cations is visible.
- Regenerate injection: Choose the right regenerant and regenerate the failed anion and cation resins. Caustic soda (NaOH) is a commonly used regenerant for anion resins and hydrogen chloride (HCl) is the regenerant for the cation resins. Inject the NaOH from the top of the vessel and collect from the middle collector. And inject the HCl at the bottom of the line and collected from the middle collector. Usually, the injection time should be 20-30 minutes until all resins are regenerated.
- Slow rinsing: Continuously rinse the mixed bed with clean water flow to displace the regenerants and remove any impurities and deposits from the water that may have accumulated on the resin surface.
- Draining: before occurring air mixing, drain the water in the vessel to help to completely mix of cation and anion resins.
- Mixing: Mix the cation and anion resins with nitrogen, fresh air or other airflow and the mixing time can be 5–15 minutes. Fill water from the top of the vessel and it will take some minutes. To prevent the disturbance of the mixing of resins, you can use NaOH diluted water in the first few minutes.
- Fast rinsing: It is the final step of regeneration, so it is also called final rinsing. The resin is thoroughly rinsed with deionized water to ensure that any residual regenerant is completely removed, avoiding contamination of the treated water. After that, the cation and anion resins are mixed evenly at the vessel and restored to the original condition and ready to serve.
Features
- High Deionization Efficiency: The combination of cation and anion resins enables efficient removal of ions in a short period, maintaining excellent performance even under high-load conditions.
- Strong Adaptability: Mixed bed resins are adaptable to a wide range of water quality conditions and flow rates, making them suitable for a broad range of industrial applications.
- Space-Saving Design: Due to their highly efficient deionization capability, mixed bed resin treatment systems are typically compact in size, helping clients save on equipment space and investment costs.
- High Water Purity: Mixed bed resins can produce ultrapure deionized water that meets or exceeds industry standards, ensuring the highest water quality for demanding applications.
Applications
Mixed bed resins are used across various industries, including:
- Electronics Industry: In semiconductor manufacturing and other electronic production processes, mixed bed resins are used to produce ultrapure water, ensuring product quality and reliability.
- Pharmaceutical Industry: Mixed bed resins are used in the preparation and purification of water for pharmaceutical production, ensuring safe and compliant water quality standards as per pharmacopeia requirements.
- Food & Beverage Industry: Mixed bed resins remove impurities, odors, and harmful compounds from water used in beverage production, ensuring the final product is of the highest quality and safety.
- Industrial Wastewater Treatment: Mixed bed resins are used in treating ion-laden industrial wastewater, effectively removing ions and reducing environmental pollution while facilitating resource recovery.