
Organic wastewater refers to wastewater containing organic substances, commonly originating from industrial production, agricultural activities, and domestic sewage. Due to the complex composition of organic wastewater, it poses severe pollution risks to water bodies and the environment, making its treatment particularly crucial. Resins, as efficient wastewater treatment materials, play a significant role in organic wastewater treatment due to their excellent adsorption properties and ion exchange & regeneration capabilities.

In organic wastewater treatment, the adsorption and ion exchange resins are adopted to remove heavy metal ions, organic pollutants and recover valuable substances.
For better performance, we provide the LWT Series, which is specially designed for organic wastewater treatment.
The ion exchange resins or adsorption resins are used for heavy metal removal, organic pollution removal and valuable substances recovery. They performs well due to their excellent ion exchange capacity and adsorption resins.
It adopts ion exchange resins, which can effectively remove heavy metal ions (e.g., lead, cadmium, copper) from wastewater through ion exchange mechanisms. These resins have negatively charged surfaces that attract positively charged metal ions.
For cation exchange resins, when wastewater containing lead ions (Pb2+) contacts the resin, the sodium ions (Na+) on the resin exchange with lead ions:
R-Na+ + Pb2+ → R-Pb2+ + Na+
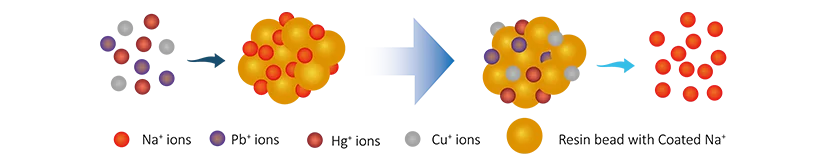
In that,
Lead ions are effectively removed, improving water quality.
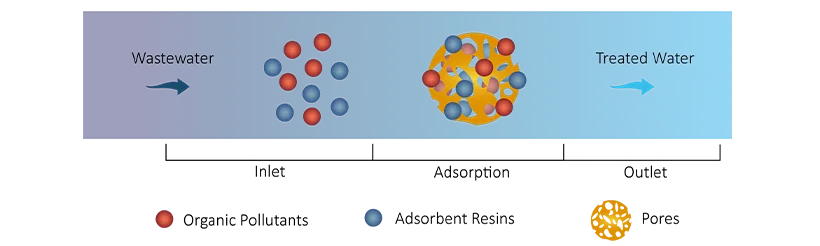
It adopts adsorption resins, which can remove organic pollutants such as benzene and ketones from water through physical or chemical adsorption. Their high surface area and specific pore structures enable effective capture of organic molecules.
For polystyrene adsorption resins, when organic pollutants (like benzene) contact the resin, benzene molecules adsorb onto the resin surface via van der Waals forces:
C6H6 (aq) + R ⇌ C6H6 (adsorbed) + R
In that,
The concentration of benzene in the water decreases, achieving the removal of organic pollutants.
It adopts resins, which possess selective adsorption capabilities for specific metal ions, making them suitable for recovering valuable metals such as gold and silver from wastewater. By controlling pH and other operational conditions, selectivity for specific metals can be enhanced.
In the recovery of gold (Au), commonly used resins contain amine or thiol groups. When gold ions contact the resin, they form a coordination compound:
Au3+ + R-SH → R-S-Au + 2H+
In that,
Gold ions are effectively adsorbed and transformed into a recoverable form, reducing resource waste.
Industry
Specific Applications

No matter guides, inquiry or assistance, our experts are ready to serve you.