
Global water scarcity is worsening, especially in industrially concentrated areas, where the demand for water resources is continually increasing. Traditional water treatment and discharge methods are inadequate to meet the needs of modern industry and societal development, while water reuse technology plays an essential role in addressing this issue. During water reuse technology, resins plays important roles to remove heavy metals, organic pollutants, Ammonia Nitrogen and Nitrates. They can help to improve water quality and provide qualified reusable water.

As we all know, the resins can be divided into ion exchange resins and adsorption resins, and the ion exchange resins contains SAC, WAC, SBA, WBA types. During water reuse treatment, the strong acid resins (SAC), chelating resins and mocroporous adsorption resins are adopted to complete water reuse treatment task.
In the wastewater reuse treatment process, resins play a significant role in removing heavy metals, organic pollutants, softening water, and eliminating ammonia nitrogen and nitrates. Different pollutants require different types of resins. The following outlines their specific applications in water reuse, including functions, types of resins, working principles, and chemical reactions.
Industrial wastewater often contains heavy metals such as copper, lead, zinc, and cadmium, which are harmful to the environment and human health and challenging to remove through biological treatment. Resins can effectively eliminate these metal ions through chemical adsorption or ion exchange.
In ion exchange, charged groups on the resin surface exchange with heavy metal ions in wastewater, binding the metal ions to the resin while releasing hydrogen or sodium ions. Chelating resins firmly adsorb metal ions by forming chemical bonds within the resin matrix:
R-H+ + Cu2+ → R-Cu2+ + 2H+

In that,
Some industrial wastewater contains refractory organic compounds (e.g., phenols, dyes), which not only degrade water quality but may also produce odors and discoloration. Adsorption resins can remove these organic substances through physical adsorption, ensuring the water is clear and meets reuse standards.
Macroporous resins remove organic pollutants through physical adsorption. Their large surface area and porous structure provide adsorption sites for organic molecules, where hydrophobic interactions between the organic compounds and the resin’s surface retain the molecules on the resin:
Corganic + R → R-Cadsorbed
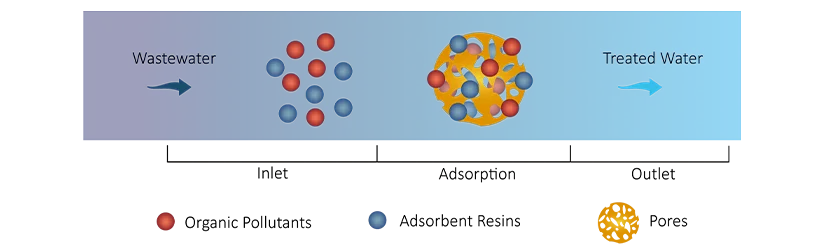
In that,
Organic molecules adhere to the pores or surface of the resin through intermolecular forces.
Some industrial wastewater contains high levels of calcium and magnesium ions, increasing water hardness. These hardness ions can cause scaling in equipment pipelines, affecting system performance. Softening the water through resins prevents scaling and ensures normal equipment operation.
During water softening, sodium ions on the cation exchange resin surface exchange with calcium and magnesium ions in the water, removing hardness ions and softening the water:
2R-Na + Ca2+ → R2Ca + 2Na+
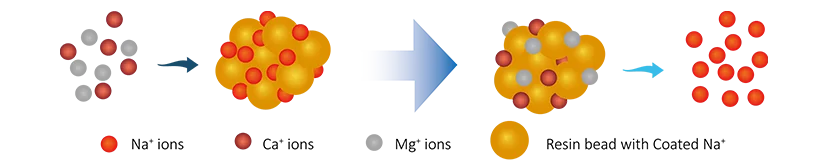
In that,
The resin removes hardness ions by exchanging sodium ions for calcium ions, achieving a softening effect.
Ammonia nitrogen and nitrates are common inorganic pollutants in wastewater, particularly in effluents from electronics and semiconductor industries. Removing these pollutants helps reduce the risk of water eutrophication and decreases the ecological impact of wastewater.
Cation exchange resins exchange with ammonia nitrogen ions in wastewater, binding ammonia nitrogen to the resin. Anion exchange resins remove nitrate ions through an exchange process:
Removal of Ammonia Nitrogen:
R-H + NH4+ → R-NH4 + H+
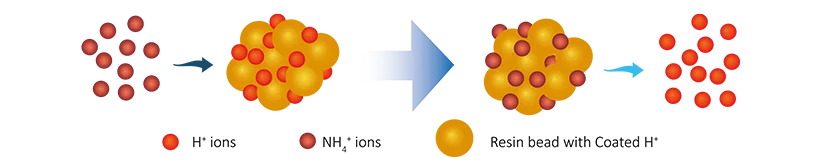
In that,
In this exchange process, the resin adsorbs the target pollutant ions, purifying the water.
Removal of Nitrate Ions:
R-Cl + NO3- → R-NO3 + Cl-
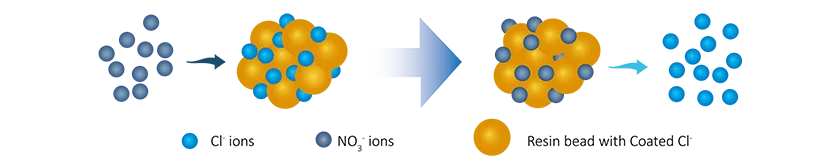
In that,
In this exchange process, the resin adsorbs the target pollutant ions, purifying the water.

No matter guides, inquiry or assistance, our experts are ready to serve you.