Water dealkization is important and is the pre-treatment for boiler. It can remove carbonate alkalinity in the feed water before it reaches the boiler. In boiled water, the first limit chemistry parameter is alkalinity. Through reducing alkalinity, it can help to decrease blowdown volumes, increase the concentration cycles and decrease energy consumption. Here we will introduce the alkalinity and why we need to reduce alkalinity and how to produce dealkalization water.
Alkalinity refers to the ability of water to neutralize acids, primarily determined by the concentration of carbonate (CO32-), bicarbonate (HCO3-), and hydroxide (OH-) ions present in the water. It reflects the total amount of substances in the water that can act as acid-base buffers and is typically measured in ppm (parts per million) or mg/L.
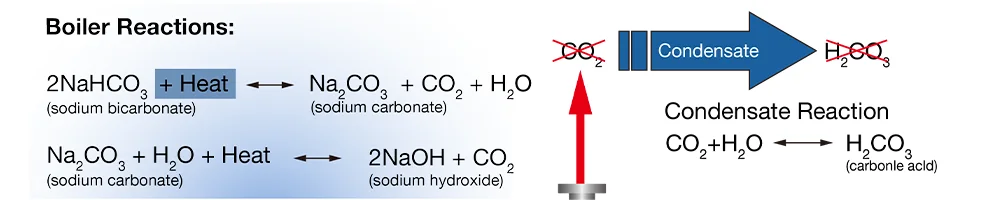
Naturally occurring alkalinity in the raw water comes in the form carbonate and bicarbonate. When alkalinity enters the boiler, it breaks down into OH- and CO2. CO2 (gas) are discharged with the steam and forms carbonic acid as the steam condenses (pH<6.0). it the water with alkalinity is untreated, low pH water can corrode the condensate network and pipelines.

Alkaline water can have various negative impacts on industrial production:
Water dealkalization main adopt ion exchange principles, that is remove the carbonate and bicarbonate ions with SAC, WAC or SBA ion exchange resins to achieve water dealkalization. There are three major method to complete water dealkalization, here we will introduce them for you one by one and give you a comparison to help you find your suitable system and choose the right ion exchange resins.
Chloride Anion Dealkalization. Choride cycle method is just like the water softening process, which exchange the harness ions (Ca+, Mg+, etc.) with Na+ ions in ion exchange. Choride cycle system adopts a strong anion base (SBA) resins to complete ion exchange. Alkalized water with carbonate and bicarbonate enters the tank and passes through the ion exchange resins, the carbonate and bicarbonate are exchanged with Choride ions on the resins. Then the alkalinity is removed and only remain Choride ions in the water. Once the resins are staturated, they need to be regenerated with sodium chloride (NaCl) or salt-caustic combination (NaOH) solution. In this system, it is commonly used combined with SAC (Strong Acid Cation) resins for softening.


Split Stream Dealkalization. This method utilizes two parallel strong acid cation (SAC) reactors. One reactor operates in sodium (Na+) form, removing hardness while retaining 100% of alkalinity, while the other operates in hydrogen (H+) form, removing all alkalinity but remaining free mineral acidity (FMA). In the nex step, these two streams blend, FMA in the hydrogen cation resins effluent coverts sodium carbonate and bicarbonate alkalinity in the sodium cation resins effluent to carbonic acid. Refer to the following reaction:

Then, the generated carbonic acid dissociates into water (H2O) and carbon dioxide (CO2), and they will be delivered into degassifier to remove carbon dioxide through a countercurrent air stream. The final water alkalinity can be controlled by managing the percentage of each mixed water flow.

Comparative Summary
| Method | Advantages | Disadvantages | Application Scenarios |
|---|---|---|---|
| Chloride Cycle Dealkalization | Effectively removes alkalinity; suitable for industrial applications | Regeneration may introduce chloride ions; management required | Industrial water treatment; prevents boiler scaling |
| Weak Acid Dealkalization | Significant cost advantages; high-quality effluent; no extra ions introduced | Relatively slow processing; limited applicability | Situations with high hardness-to-alkalinity ratios |
| Split Stream Dealkalization | Flexible adjustments to effluent alkalinity; high stability and adaptability | Complex system design; requires high control and monitoring | Handling varying water quality; adaptable treatments |
Choosing the appropriate dealkalization method is crucial for optimizing water treatment effectiveness, reducing production costs, and ensuring product quality. By understanding the advantages and disadvantages of different processes, industrial enterprises can make informed decisions based on their specific needs.