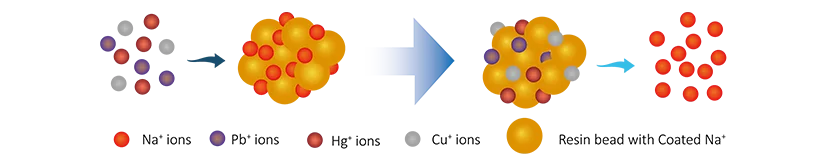Inorganic Waste Water Treatment with Chelating Resins
Inorganic wastewater refers to the wastewater containing heavy metals. If the heavy metal contaminated wastewater was discharge to environment, it will threat human health and the ecosystem. Heavy metal ions removal is the key to inorganic wastewater treatment. Additional, value metal recycling helps optimize resources and reduce discharge. We provide high selective chelating resins to help to remove heavy metals, such as silver (Ag), iron (Fe), manganese (Mn), molybdenum (Mo), boron (B), calcium (Ca), antimony (Sb), cobalt (Co), etc.; and they can help to recycling value metals, such as manganese (Mn), nickel (Ni), Copper (Cu), cobalt (Co), etc.
Resin Options
We supply the high selective chelating resins for inorganic wastewater treatment applications. Chelating resins can effectively remove harmful heavy metal ions through the process of adsorption and ion exchange. And it can help to recycle value metals as well.
How Resin Based Inorganic Water Treatment Works?
Ion exchange resin acts like a "sponge", with porous structure on its surface that can absorb ions. It has fixed anions and exchangeable cations (like sodium or hydrogen ions). The working principle is as follows:
- Flow of Wastewater: When wastewater containing heavy metals passes through the resin, the heavy metal ions (such as lead or cadmium) in the wastewater exchange with the cations on the resin.
- Ion Replacement: The heavy metal ions attach to the resin while the resin releases its cations (like sodium) into the wastewater. It’s like a "swap game" where the heavy metal ions are secured to the resin and the resin’s cations are released into the water.
- Removal of Heavy Metals: Through this process, the heavy metal ions in the wastewater are absorbed by the resin, reducing harmful substances in the water and purifying it.
- Regeneration: Once the resin has absorbed a certain amount of heavy metals, it needs to be regenerated, usually by washing it with acid or alkali to remove the absorbed metals, restoring its ability to adsorb.
The reaction is as follows:
R-M+ + W+ ↔ R-W+ + M+
For example,
When a sodium ion (Na+) on the resin is replaced by a lead ion (Pb2+) from the wastewater, it can be written as:
R-Na+ + Pb2+ ↔ R-Pb2+ + Na+
In that:
- R-Na+, represents the ion-exchange resin with a fixed anion (R-) and an exchangeable cation (Na+)
- Pb2+, represents the heavy metal cation in the wastewater
Industries and Specific Applications of Inorganic Wastewater Treatment
Industry
- Metallurgy and metal processing: Wastewater containing metals generated from processes such as electroplating, smelting, and forging.
- Chemical industry: Wastewater from chemical synthesis, pharmaceuticals, and dye production processes.
- Mining: Wastewater from ore washing and refining processes.
- Pharmaceutical and food processing: Wastewater generated from the use of chemical agents and additives in these processes.
Typical Application for Resource
- Removal Hg in electroplating
- Removal Cr in electroplating
- Removal Pb in electroplating
- Removal As in hydrometallurgy
- Removal Ca in RO concentrated water
- Removal Mg in RO concentrated water
- Recycling Mn in catalyst
- Recycling Cu in catalyst
- Recycling Co in catalyst
- Recycling Ni in catalyst
Related Information
What is inorganic waters?
Inorganic wastewater refers to wastewater that primarily contains inorganic substances, which typically do not contain carbon-hydrogen bonds (C-H), in contrast to organic wastewater. The main components of inorganic wastewater include the following categories:
- Heavy metal ions: Such as lead (Pb), cadmium (Cd), copper (Cu), zinc (Zn), and chromium (Cr), which usually originate from industries such as electroplating, metallurgy, mining, and chemicals.
- Salts: Such as chlorides (Cl-), sulfates (SO42-), hydroxides (OH-), and carbonates (CO32-), commonly found in wastewater from chemical, pharmaceutical, and electroplating industries.
- Inorganic acids and bases: Such as sulfuric acid (H2SO4), hydrochloric acid (HCl), and sodium hydroxide (NaOH), which are often used in industrial production processes.
- Ammonia nitrogen: Such as ammonium ions (NH4+), primarily sourced from agriculture and certain industrial processes.
- Other inorganic substances: Such as silica (SiO2), fluorides (F-), and phosphates (PO43-), which may come from mining and metallurgy.
What is the harm of heavy metals of inorganic wastewater?
Heavy metal pollution in wastewater is a significantly serious environmental problem. If untreated wastewater containing heavy metals was discharged into the environment can contaminate aquatic life and agricultural crops. Heavy metals can then enter the food chain, be absorbed through the human body and potentially cause serious health issues due to their toxicity.